
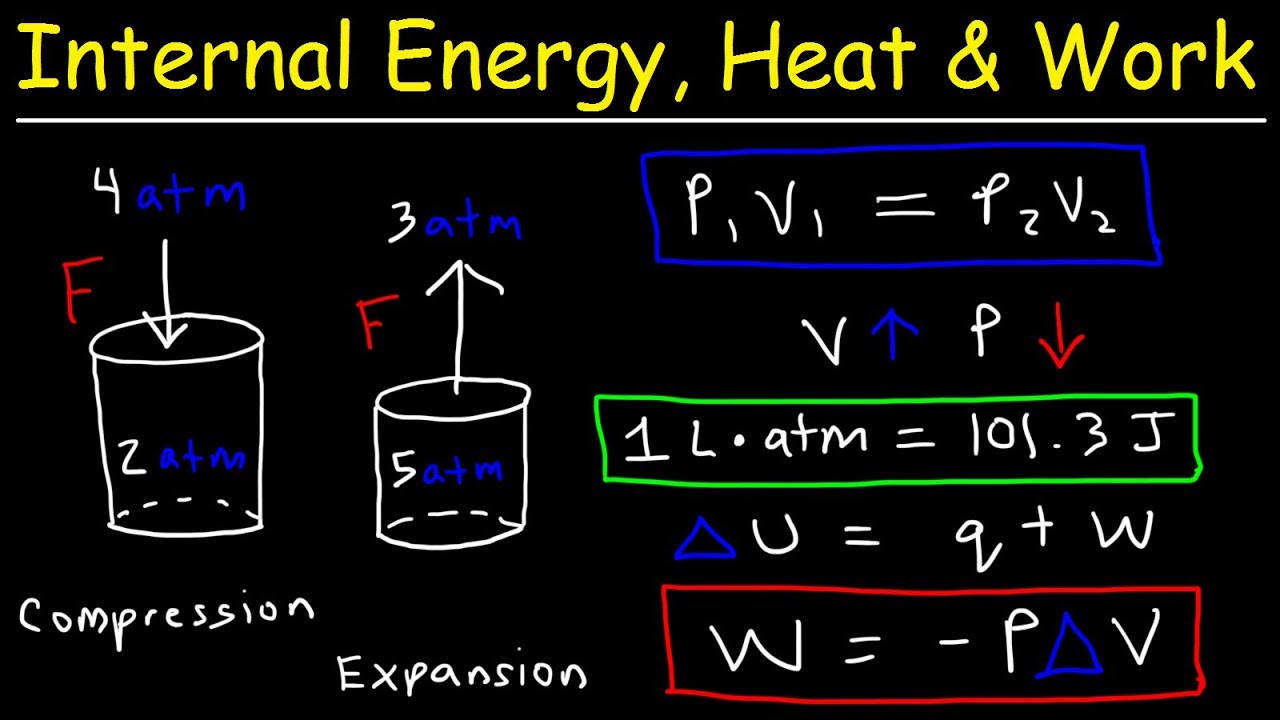
This is in distinction to heat energy carried into or out of the system in the form of transfers in the microscopic thermal motions of particles. Work refers to forms of energy transfer, which can be accounted for in terms of changes in the macroscopic physical variables of the system, for example energy which goes into expanding the volume of a system against an external pressure, by say driving a piston-head out of a cylinder against an external force. The modern day definitions of heat, work, temperature, and energy all have connection to this experiment.Īccording to the First Law of Thermodynamics, it is useful to separate changes to the internal energy of a thermodynamic system into two sorts of energy transfers. Joule estimated a mechanical equivalent of heat to be 819 ft Using these values, Joule was able to determine the mechanical equivalent of heat. Both the temperature ∆T change of the water and the height of the fall ∆h of the weight mg were recorded.
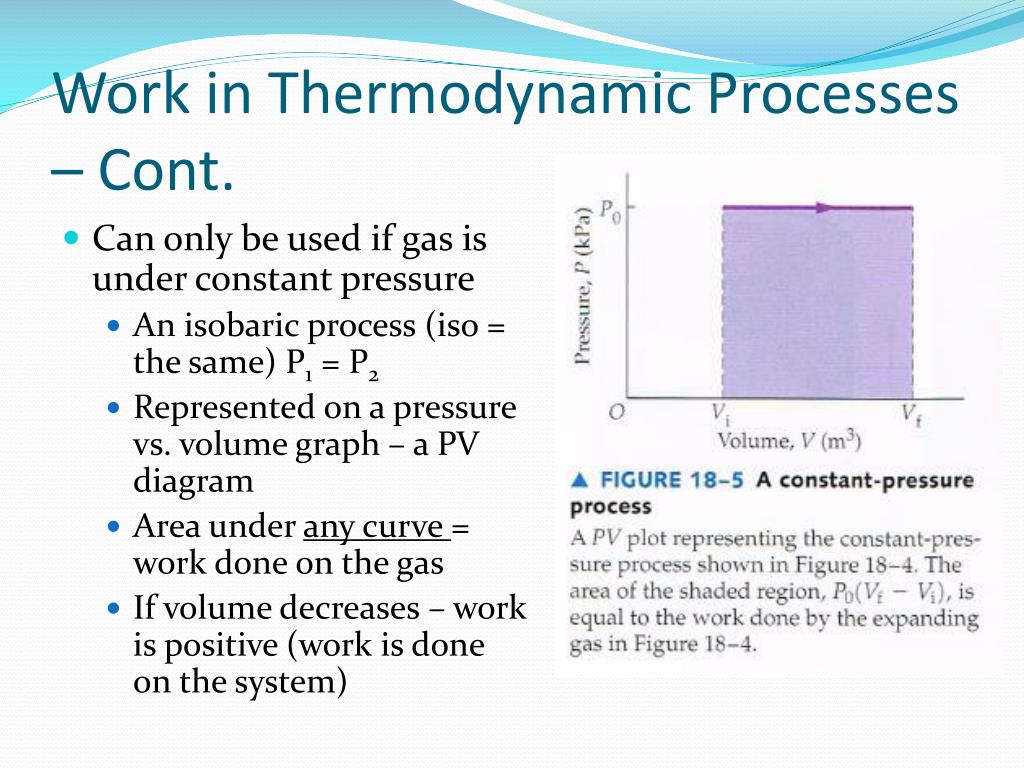

In this experiment, the friction and agitation of action the paddle-wheel on the body of water caused heat to be generated which, in turn, increased the temperature of water. In this work, he reported his best-known experiment, that in which the work released through the action of a "weight falling through a height" was used to turn a paddle-wheel in an insulated barrel of water. In 1845, the English physicist James Joule read a paper On the mechanical equivalent of heat to the British Association meeting in Cambridge.


 0 kommentar(er)
0 kommentar(er)
